Abstract
Background: Standard therapy of acute promyelocytic leukemia (APL) has long relied on the combination of All- trans -retinoic acid (ATRA) and chemotherapy. The introduction of arsenic trioxide (ATO) into APL treatment resulted in similarly high remission and survival rates coupled with significantly reduced myelosuppression. Recent results of the APL0406 trial by the GIMEMA-AMLSG-SAL study groups showed that the combination of ATRA and ATO is superior to standard ATRA and chemotherapy (CHT) in front-line therapy of low/intermediate-risk APL. The implications of these results for the clinical practice of APL patients in Germany have been uncertain given the fact that ATO has only recently been licensed for front-line therapy in APL subgroups.
Aim: In order to provide a picture of the clinical reality of APL patient care in Germany an intergroup APL registry (National acute promyelocytic leukemia (APL) observational study, NAPOLEON) was recently initiated by 4 AML study groups.
Methods: Eligible patients are adults at least 18 years of age with newly diagnosed or relapsed APL within the first year after diagnosis. Here we report the analysis on the series of patients prospectively enrolled into this registry. The study was conducted in accordance with the Declaration of Helsinki, received IRB approval by all participating centers and was registered at ClinicalTrials.gov (NCT02192619).
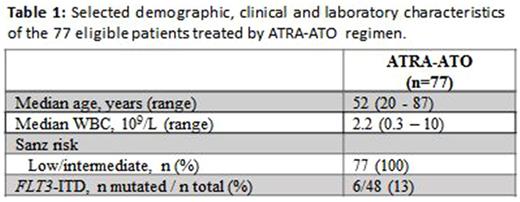
Results: As of August 1st 2017, 150 patients (median age 54 years; range 19-87) with newly diagnosed APL have been included into the study. Among all patients 67% (n=100) were low/intermediate-risk according to the Sanz score. Out of these patients 77% (n=77) received an ATO-ATRA based induction regimen followed by a median of 4 courses of ATO-ATRA consolidation (according to the APL 0406 study).Of 76 patients treated by ATRA-ATO regimen that were evaluable for response to induction, 75 (99%) patients achieved a complete remission (CR). One patient died within 30 days of therapy resulting in an early death rate of 1%. After a median follow-up of 14 months, event-free survival, cumulative incidence of relapse and overall survival at 12 months for these patients were 97%, 2% and 97%, respectively. The therapy was well tolerated and no new safety signals have been obtained.
Conclusion: These real life data from a prospective German registry provide further evidence for the safety and sustained anti-leukemic efficacy of ATRA-ATO in low/intermediate-risk APL. These results further support ATRA-ATO as the new standard of care in this clinical setting.
Platzbecker: Acceleron: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding. Götze: Celgene: Honoraria; Novartis: Honoraria; Amgen: Honoraria; Abbvie: Honoraria; BMS: Honoraria. Hänel: Roche: Honoraria; Novartis: Honoraria. Greil: Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support, Research Funding; Novartis, Celgene: Research Funding; Takeda: Honoraria, Research Funding; BMS, Amgen: Honoraria. Greiner: Bristol-Myers Squibb: Research Funding. Hochhaus: Novartis: Research Funding; BMS: Research Funding; MSD: Research Funding; Incyte: Research Funding; Pfizer: Research Funding; ARIAD: Research Funding. Fiedler: Amgen: Patents & Royalties; Amgen, Gilead, GSO, Teva, Jazz Pharmaceuticals: Other: Support for meeting attendance; Amgen, ARIAD/Incyte: Membership on an entity's Board of Directors or advisory committees; Amgen, Pfizer: Research Funding. Hiddemann: Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Thiede: Roche: Consultancy; Bayer: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Agendix: Employment. Brummendorf: Takeda Pharmaceuticals International Co: Consultancy, Research Funding. Dohner: Novartis: Honoraria, Research Funding. Rollig: Janssen: Research Funding; Bayer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal